

Title and structured summary
Item 1b: Structured summary of trial design and methods, including items from the World Health Organization Trial Registration Data Set.
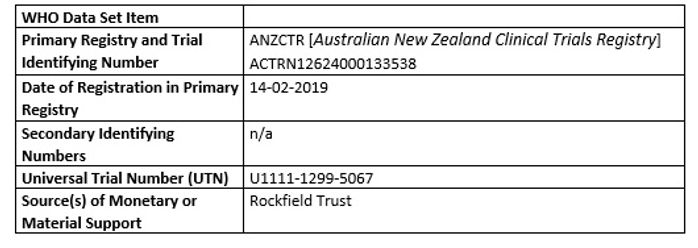


Administrative information
Open Science
Introduction
Methods: Patient and public involvement, trial design
Methods: Participants, interventions, and outcomes
Methods: Assignment of interventions
Methods: Data collection, management, and analysis
Methods: Monitoring
Ethics
Explanation
A structured summary provides an accessible overview of the planned trial for users of the protocol. The World Health Organization (WHO) Trial Registration Data Set should be included in the protocol to serve as a brief structured summary of the trial. The WHO Trial Registration Data Set defines the minimum information to be included in a trial registry in order for a trial to be considered fully registered (Item 4) [30]. These standards are supported by the International Committee of Medical Journal Editors (ICMJE), other journal editors, and legislation in many countries [31-33].
Reporting of the Data Set in the protocol can facilitate the prospective transfer of accurate information to a trial registry (i.e., before inclusion of the first trial participant). This can also signal the need for updates to the registry record when associated protocol sections are amended – thereby promoting consistency of information between the protocol and registry.
Summary of key elements to address
Relevant items from the WHO Trial Registration Data Set: [30]
-
Primary registry and trial identifying number
-
Secondary identifying numbers
-
Source(s) of monetary or material support
-
Primary sponsor
-
Contact for public queries
-
Contact for scientific queries
-
Public title
-
Scientific title
-
Countries of recruitment
-
Health condition(s) or problem(s) studied
-
Intervention(s)
-
Key inclusion and exclusion criteria
-
Study type
-
Date of first enrolment (planned)
-
Sample size
-
Primary outcome(s)
-
Key secondary outcome(s)
-
Ethics review
-
Individual trial participant data sharing statement